Pfizer mRNA Vaccine: Risks of Pulmonary Embolism, Myocarditis, Blood Clots, Immune Thrombosis. Finally Released the FDA Surveillance Data

by Fabio Giuseppe Carlo Carisio
In the lates weeks the British Medical Journal blamed the Food and Drug Administration because had concealed the result of a big study of active pharmacovigilance, so not only based on individual and free reports to database (EudraVigilance managed by EMA in European Union and VAERS by CDC in US), instead focused also on a follow-up some vaccinees.
The statistical research named “Surveillance of COVID-19 vaccine safety among elderly persons aged 65 years and older” was finally released by FDA and published on December, 1, 2022 by specialistic Journal of Vaccine and Elsevier by Science Direct.
The first signatory is Hui-Lee Wong, Associate Director for Innovation and Development Office of Biostatistics and Epidemiology, Center for Biologics Evaluation of US Food and Drug Administration, Silver Spring, MD, USA. The study is focused on data covering 30,712,101 elderly persons.
British Medical Journal’s BOMB: “US FDA Hides Data on Serious Adverse Events after Covid Vaccines”
Below we report the Abstract in which FDA confirmed the alarms launched by many other studies since two years and more about the risk of 4 serious adverse reactions sometimes lethal: pulmonary embolism, acute myocardial infarction, disseminated intravascular coagulation, and immune thrombocytopenia.
These are the same side effects denounced by Gospa News in many scientific articles based on clinical researches or literary study.
The huge disturbing issue is on the pulmonary embolism because the danger of this autoimmune damage with mRNA sera was highlighted by two Chinese university on October, 2020 when the scientists asked to stop the advanced trial clinic on human guineas pig and to restart the research from the beginning on mice.
Instead this study was ignored by scientific community and FDA, CDC, EMA and in Italy AIFA went on with emergency use authorization under press ion of the will of Western governments, the US, EU, German and Italian ones above all, which was lobbied by Bill Gates, Pfizer and GSK (Pfizer partner) as we demonstrated in many investigations of WuhanGates cycle.
How many people was killed by these choices?
However, there are still very important limitations in this FDA study. First of all, the study reports statistics concerning only the mRNA vaccine produced by the pharmaceutical company Pfizer in New York and not the similar one from Moderna in Cambridge.
This may have been determined by the precise desire not to point the finger at Moderna’s biotechnology which was financed directly by Gates and by the US government through three instruments: Operation Warp Speed (which Pfizer renounced), grants from the military agency DARPA of the Pentagon and those of Anthony Fauci’s NIAID.
As we well know, thanks to this money, Cambridge’s Big Pharma managed to patent the active principle of its anti-Covid vaccine in March 2019, or nine months before the Covid-19 pandemic.
Another somewhat suspicious issue is that the FDA, after having approved bivalent boosters even for 6-month-old babies, continues to arbitrarily believe that the risk of these very serious side effects is lower than that deriving from a symptomatic infection with Covid-19 although by now any doctor on earth is aware that it can be easily treated with anti-inflammatories, antivirals and antibiotics if administered at the first symptoms (and not after 72 as supported by the criminal protocol of the Ministry of Health in Italy).
The third element that makes this research of little use in understanding the real seriousness of the adverse reactions of Pfizer-Biontech’s Comirnaty gene serum derives from the fact that it is limited to the over 65s while the more serious side effects and unexplained sudden illnesses are even more common among the under 4os and even more so among the under 20s.
That’s why, although the FDA study confirms what has already been claimed by thousands of serious doctors and scientists for two years now. it still proves to be too much in favor of Big Pharma which has been influencing the choices of Western governments for years.
Below is the summary and a commentary article by The Epoch Time science journalist Zachary Stieber.
Fabio Giuseppe Carlo Carisio
© COPYRIGHT GOSPA NEWS
prohibition of reproduction without authorization
follow Gospa News on Telegram
MAIN SOURCES
GOSPA NEWS – WUHAN-GATES INQUIRIESGOSPA NEWS – COVID-19 & VACCINES KILLER DOSSIER
Abstract
Background
Monitoring safety outcomes following COVID-19 vaccination is critical for understanding vaccine safety especially when used in key populations such as elderly persons age 65 years and older who can benefit greatly from vaccination. We present new findings from a nationally representative early warning system that may expand the safety knowledge base to further public trust and inform decision making on vaccine safety by government agencies, healthcare providers, interested stakeholders, and the public.
Methods
We evaluated 14 outcomes of interest following COVID-19 vaccination using the US Centers for Medicare & Medicaid Services (CMS) data covering 30,712,101 elderly persons. The CMS data from December 11, 2020 through Jan 15, 2022 included 17,411,342 COVID-19 vaccinees who received a total of 34,639,937 doses. We conducted weekly sequential testing and generated rate ratios (RR) of observed outcome rates compared to historical (or expected) rates prior to COVID-19 vaccination.
Findings
Four outcomes met the threshold for a statistical signal following BNT162b2 vaccination including pulmonary embolism (PE; RR = 1.54), acute myocardial infarction (AMI; RR = 1.42), disseminated intravascular coagulation (DIC; RR = 1.91), and immune thrombocytopenia (ITP; RR = 1.44). After further evaluation, only the RR for PE still met the statistical threshold for a signal; however, the RRs for AMI, DIC, and ITP no longer did. No statistical signals were identified following vaccination with either the mRNA-1273 or Ad26 COV2.S vaccines.
Interpretation
This early warning system is the first to identify temporal associations for PE, AMI, DIC, and ITP following BNT162b2 vaccination in the elderly. Because an early warning system does not prove that the vaccines cause these outcomes, more robust epidemiologic studies with adjustment for confounding, including age and nursing home residency, are underway to further evaluate these signals. FDA strongly believes the potential benefits of COVID-19 vaccination outweigh the potential risks of COVID-19 infection.

Pfizer’s COVID-19 Vaccine Linked to Blood Clotting: FDA
by Zachary Stieber – originally published by The Epoch Time – All links to Gospa News articles have been added aftermath
Pfizer’s COVID-19 vaccine has been linked to blood clotting in older individuals, according to the U.S. Food and Drug Administration (FDA).
FDA researchers, crunching data from a database of elderly persons in the United States, found that pulmonary embolism—blood clotting in the lungs—met the initial threshold for a statistical signal and continued meeting the criteria after a more in-depth evaluation.
Three other outcomes of interest—a lack of oxygen to the heart, a blood platelet disorder called immune thrombocytopenia, and another type of clotting called intravascular coagulation—initially raised red flags, researchers said. More in-depth evaluations, such as comparisons with populations who received influenza vaccines, showed those three as no longer meeting the statistical threshold for a signal.
Researchers looked at data covering 17.4 million elderly Americans who received a total of 34.6 million vaccine doses between Dec. 10, 2020, and Jan. 16, 2022.
The study was published by the journal Vaccine on Dec. 1.
The FDA said it was not taking any action on the results because they do not prove the vaccines cause any of the four outcomes, and because the findings “are still under investigation and require more robust study.”
Pfizer did not respond to a request for comment.
The initial results of the safety monitoring detected an increased risk of four events, the FDA announced on July 12, 2021. They were the same four outlined in the new paper, which is the first update the agency has given on the matter since its announcement.
As of Jan. 15, 2022, 9,065 cases of a lack of oxygen to the heart—known as acute myocardial infarction—were detected, researchers revealed in the new study. As of the same date, 6,346 cases of pulmonary embolism, 1,064 cases of immune thrombocytopenia, and 263 cases of the coagulation were detected.
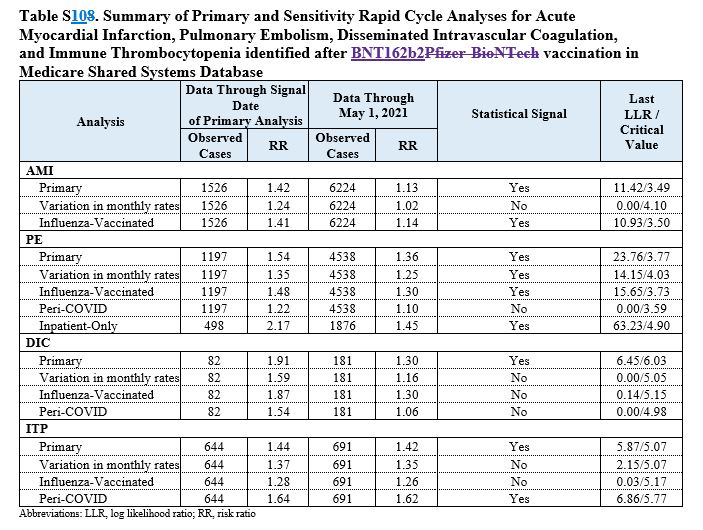
The primary analysis showed a safety signal for all four outcomes. Researchers tried adjusting the numbers by using different variables. For instance, at one point they adjusted for the variation of background rates, or the rates of each outcome in the general population prior to the pandemic. After certain adjustments—not all—the myocardial infarction, immune thrombocytopenia, and intravascular coagulation ceased being statistically significant.
Pulmonary embolism, though, continued to be statistically significant, the researchers said. Pulmonary embolism is a serious condition that can lead to death.
Limitations of the study included possible false signals and possible missed signals due to factors such as parameters being specified wrongly.
The conditions that didn’t trigger a signal included stroke, heart inflammation, and appendicitis.
The signals were detected only after Pfizer vaccination. Analyses for signals after receipt of the Moderna and Johnson & Johnson vaccines did not show any concerns.
Moderna and Johnson & Johnson did not respond to requests for comment.
Side Effects
All three vaccines have been linked to a number of side effects. Heart inflammation is causally linked to the Moderna and Pfizer shots, experts around the world have confirmed, while Johnson & Johnson’s has been associated with blood clots.
Other conditions, such as pulmonary embolism, have been reported to authorities and described in studies, though some papers have found no increase in risk following vaccination.
Approximately 4,214 reports of post-vaccination pulmonary embolism, including 1,886 reports following receipt of Pfizer’s vaccine, have been reported to the U.S. Vaccine Adverse Event Reporting System as of Dec. 9.
As of the same date, 1,434 reports of post-vaccination myocardial infarction, including 736 following receipt of Pfizer’s vaccine; 469 reports of post-vaccination immune thrombocytopenia, including 234 following receipt of Pfizer’s vaccine; and 78 reports of post-vaccination intravascular coagulation, including 42 after receipt of Pfizer’s vaccine, have been reported.
Reports to the system can be made by anybody, but most are lodged by health care workers, studies show. The number of reports are an undercount, according to studies.
The new study states that the FDA “strongly believes the potential benefits of COVID-19 vaccination outweigh the potential risks of COVID-19 infection.” No evidence was cited in support of the belief.
The FDA is set to meet with its vaccine advisory panel in January 2023 about the future of COVID-19 vaccines, as the vaccines have been performing much worse against Omicron and its subvariants.
McCullough told The Epoch Times: “A shortcoming of the CMS surveillance system is that it did not capture prior and subsequent SARS-CoV-2 infection which accentuate the cumulative risk of COVID-19 vaccination. Given the large number of individuals who have been vaccinated, the population attributable fraction of medical problems ascribed to the vaccines is enormous. I have concerns over the future burden to the healthcare system as a consequence of mass indiscriminate COVID-19 vaccination.”
by Zachary Stieber – originally published by The Epoch Time – All links to Gospa News articles have been added aftermath
Zachary Stieber is a senior reporter for The Epoch Times based in Maryland. He covers U.S. and world news.




4 pensieri su “Pfizer mRNA Vaccine: Risks of Pulmonary Embolism, Myocarditis, Blood Clots, Immune Thrombosis. Finally Released the FDA Surveillance Data”