Breaking! Texas Attorney General has Sued Pfizer for Providing Adulterated Drugs to Children

Introduction by Fabio Giuseppe Carlo Carisio
Subscribe to the Gospa News Newsletter to read the news as soon as it is published
“If any company illegally took advantage of consumers during this period or compromised people’s safety to increase their profits, they will be held responsible”
Texas Attorney General Ken Paxton made these statements on May, 2023 in announcing that he had launched an investigation into whether Pfizer, Moderna and Johnson & Johnson committed fraud related to the COVID-19 vaccines.
Paxton will investigate whether the companies misrepresented the efficacy and safety of the vaccines and manipulated vaccine trial data, in violation of the state’s Deceptive Trade Practices Act.
Paxton earned credit for being one of the first magistrates in the entire West to open a formal investigation into the damage caused by Covid vaccines.
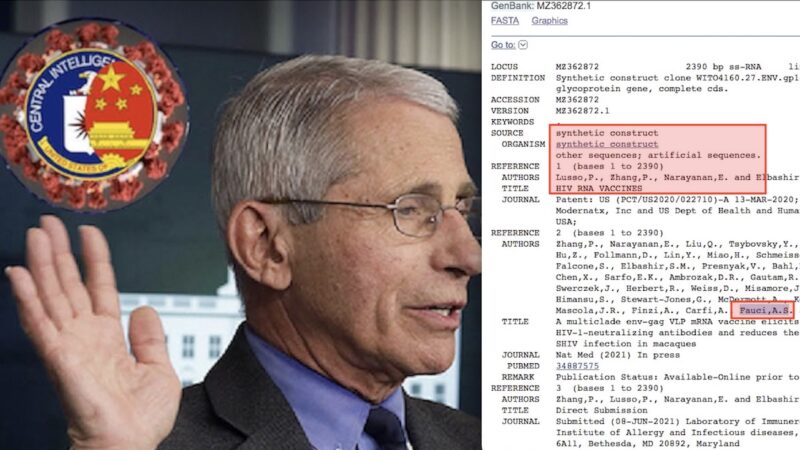
Not only that: he had also announced his intention to investigate the dangerous experiments with the Gain-of-Function viral enhancement technique made famous by Anthony Fauci’s researchthanks to funding from the Obama-Biden administration and the Pentagon with which SARS- Cov-2 would have been created in the laboratory, according to the report of the US Senate Health Commission.
But that crucial investigation into the most disturbing aspects of the pandemic was temporally stopped because Paxton has been subjected to an “impeachment” procedure by the Texas State House of Representatives and, therefore, will no longer be able to conduct this investigation.
On September the Attorney General of Texas was acquitted of 16 impeachment articles, thwarting an effort to remove him from office over allegations of corruption.
So he reinstated to office and started again his inquiry. Meanwhile he receive two petitions by private person plaintiff on other matter “for defrauding the Texas Medicaid program by providing adulterated pharmaceutical drugs to Texas children in violation of the Texas Medicaid Fraud Prevention Act” so he immediately opened one lawsuit against Big Pharma of New York, Pfizer, and other pharmaceutical companies.
Below every details.
Fabio Giuseppe Carlo Carisio
© COPYRIGHT GOSPA NEWS
prohibition of reproduction without authorization
follow Fabio Carisio Gospa News director on Twitter
follow Gospa News on Telegram
Subscribe to the Gospa News Newsletter to read the news as soon as it is published
Statements by Office of the Attorney General’s Civil Medicaid Fraud Division
Attorney General Sues Pfizer for Defrauding Texas Medicaid and Providing Adulterated Pharmaceutical Drugs to Children
All links to previous Gospa News investigations have been added aftermath, for the ties with the topics highlighted Subscribe to the Gospa News Newsletter to read the news as soon as it is published
The Office of the Attorney General’s Civil Medicaid Fraud Division has sued Pfizer, Inc., Tris Pharma, Inc. and Tris CEO Ketan Mehta for defrauding the Texas Medicaid program by providing adulterated pharmaceutical drugs to Texas children in violation of the Texas Medicaid Fraud Prevention Act, now known as the Texas Health Care Program Fraud Prevention Act (“THFPA”).

Pfizer contracted with Tris, a drug manufacturer, to produce a pediatric attention-deficit/hyperactivity disorder medication (“ADHD”), Quillivant XR. Pfizer knowingly distributed Quillivant to children on Medicaid despite the drug’s pattern of failing quality control tests due to flawed manufacturing practices. For years, Tris altered the drug’s testing method in violation of federal and state laws to ensure Quillivant passed regulatory hurdles and could continue to be sold.
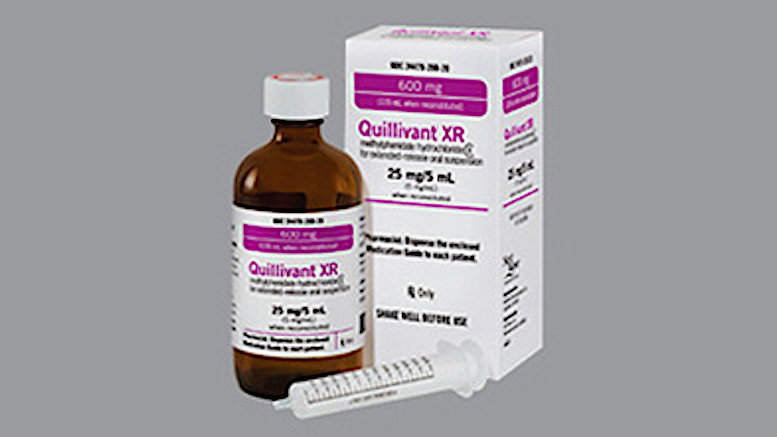
Despite knowing about these serious problems, Pfizer misrepresented to the Medicaid program that Quillivant was in compliance with federal and state law, and concealed from Medicaid decision-makers the fact that Quillivant was an adulterated drug. As a result of these misrepresentations and concealments, Pfizer and Tris obtained the benefit of taxpayer-funded Medicaid reimbursement for Quillivant.
From 2012 to 2018, Pfizer and Tris continually manipulated Quillivant testing to hide poor manufacturing practices and defraud the Texas Medicaid program. During this time, many families complained that the medication failed to work.
“I am horrified by the dishonesty we uncovered in this investigation,” said Attorney General Paxton. “Pfizer and Tris intentionally concealed and failed to disclose the issues with Quillivant to receive taxpayer funded benefits through Texas Medicaid, defrauding the state and endangering children. Our Civil Medicaid Fraud Division has done an outstanding job holding these pharmaceutical companies accountable.”
The filing explains: “At no point did Defendants warn Texas Medicaid providers or decision-makers that Quillivant had known manufacturing issues affecting its efficacy, thereby depriving the Medicaid program of the crucial information it relies on.… As a result, thousands of Texas children received an adulterated Schedule II Controlled Dangerous Substance.”
The lawsuit was initially filed under seal, but the judge has since unsealed the petition at the Attorney General’s request. To read the unsealed petition, click here.
Paxton Sues Drug Manufacturer Tris Pharma for Defrauding Texas Taxpayers
The Office of the Attorney General’s Civil Medicaid Fraud Division has sued Tris Pharma, Inc. and Tris CEO Ketan Mehta for defrauding the Texas Medicaid program by making false statements in violation of the Texas Medicaid Fraud Prevention Act, now known as the Texas Health Care Program Fraud Prevention Act (“THFPA”).
Tris manufactured a potent attention-deficit/hyperactivity disorder (“ADHD”) drug for children called Dyanavel XR and targeted Texas Medicaid with a fraudulent marketing scheme for the purpose of receiving taxpayer reimbursements through the program. In 2015, Tris launched Dyanavel into a saturated ADHD medication market and sought to differentiate the drug from competitors. To do so, Mehta and Tris pushed false claims that overstated Dyanavel’s efficacy.
Tris directed their sales representatives to deliver false and misleading messages about Dyanavel to doctors in Texas, including Medicaid doctors. Sales representatives falsely told doctors that Dyanavel worked significantly faster than other drugs and provided other unproven benefits to pediatric patients.
The filing explains that Tris’s “false and/or misleading messages regarding the efficacy of Dyanavel XR were disseminated repeatedly on thousands of sales calls to Texas Medicaid providers and decision makers.”
“The lengths to which this company and their leadership went to defraud our state and the patients taking this medication are shocking,” said Attorney General Paxton. “Pharmaceutical companies who violate the public’s trust and hurt the people of Texas will be brought to justice to the fullest extent of the law.”
The lawsuit was initially filed under seal, but the judge has since unsealed the petition at the Attorney General’s request. To read the unsealed petition, click here.
All links to previous Gospa News investigations have been added aftermath, for the ties with the topics highlighted
Subscribe to the Gospa News Newsletter to read the news as soon as it is published
MAIN SOURCES
OFFICIAL WEBSITE OF KEN PAXTON ATTORNEY GENERAL OF TEXAS
GOSPA NEWS – COVID-19, VACCINES & BIG PHARMA DOSSIER
GOSPA NEWS – WUHAN-GATES INVESTIGATIONS
https://www.gospanews.net/en/2023/11/20/bombshell-among-326-autopsies-on-vaccinated-dead-3-in-4-killed-by-mrna-jabs-university-of-michigan-study-in-eu-scientific-archive/